 |
| September 27, 2016 | Volume 12 Issue 36 |
Designfax weekly eMagazine
Archives
Partners
Manufacturing Center
Product Spotlight
Modern Applications News
Metalworking Ideas For
Today's Job Shops
Tooling and Production
Strategies for large
metalworking plants
New surface prep treatment can help bond all types of metals

The Kiel-based research team of Melike Baytekin-Gerngroß (on the left), Mark-Daniel Gerngroß, Jürgen Carstensen, and Rainer Adelung compares test results of their new bonding process in the laboratory. [Photo/Copyright: Julia Siekmann/Kiel University]
How metals can be joined depends particularly on the characteristics of their surfaces. A research team at Kiel University in Germany has discovered how they can change the surface properties of a metal and make it adherable without affecting the mechanical stability of the bulk metal piece.
This fundamentally new method is based on using an electrochemical etching process, in which the uppermost layer of a metal is roughened on a micrometer scale in a tightly controlled manner. Through this "nanoscale-sculpturing" process, metals such as aluminum, titanium, or zinc can permanently be joined with nearly all other materials. The process creates nanonscale hooked structures on the metal's surface that help make bonding incredibly strong.
The potential spectrum of applications for making "super connections" is extremely broad, ranging from metalwork in industry right through to safer implants in medical technology. The researchers' results have been published* in the journal "Nanoscale Horizons" of the Royal Society of Chemistry.
"We have now applied a technology to metals that was previously only known from semiconductors. To use this process in such a way is completely new," said Dr. Jürgen Carstensen, co-author of the paper.
"As such, we have developed a process that, unlike other etching processes, does not damage the metals, and does not affect their stability," emphasized Professor Rainer Adelung, head of the Functional Nanomaterials team at the Institute for Materials Science. "In this way, we can permanently connect metals which could previously not be directly joined, such as copper and aluminum."
Adelung also told Designfax that, "if you have two sculptured surfaces, the individual hook structures do not fit together, so a direct connection is not possible."
That means that you cannot take two treated metals' ends and push or rub them together and magically they will stick. It's the hooks that give the glue, for example, something to really hold onto.
In another interesting application, an etched metal surface of aluminum was adhered to a thermoplastic using only heat and absolutely no glue. In this case, the melted thermoplastic filled in the gaps between the nanoscale metal hooks, making the bond incredibly strong.
One secondary effect of the etching process is that the metal surface that is etched can also become water repellent (more about that later). The metal can also have its biocompatibility improved.
How does the "nanoscale-sculpturing" process work?
The surfaces of metals consist of many different crystals and grains, some of which are less chemically stable than others. These unstable particles can be specifically removed from the surface of a metal by targeted etching. The top surface layer is roughened by the etching process, creating a three-dimensional surface structure that includes undercuts that form nanoscale hooks. This changes the properties of the surface, but not of the metal as a whole. This is because the etching is only 10 to 20 micrometers deep -- a layer as thin as a quarter of the diameter of human hair. That is why the research team named the process "nanoscale-sculpturing."

A strip of aluminum that has had its surface treated with an electrochemical etching process is permanently bonded with thermoplastic by heating. [Photo/Copyright: Julia Siekmann/Kiel University]
The change due to etching is visible to the naked eye: The treated surface becomes matt. "If, for example, we treat a metal with sandpaper, we also achieve a noticeable change in appearance, but this is only two-dimensional and does not change the characteristics of the surface," explained Dr. Mark-Daniel Gerngroß of the research team on materials sciences from Kiel University. In other words, sandpapering does not help make the metal markedly more adherable.

Large metal surfaces can also be treated with nanoscale sculpturing. Although the etching is only applied to a thin layer on a micrometer scale, the resulting change is visible to the naked eye: the treated surface of the aluminum in the foreground has become matt. [Photo/Copyright: Julia Siekmann/Kiel University]
Through the etching process, a 3D structure with tiny hooks is created. If a bonding polymer is then applied between two treated metals, the surfaces interlock with each other in all directions like a three-dimensional puzzle. "These 3D puzzle connections are practically unbreakable. In our experiments, it was usually the metal or polymer that broke, but not the connection itself," said Melike Baytekin-Gerngroß, lead author of the publication.
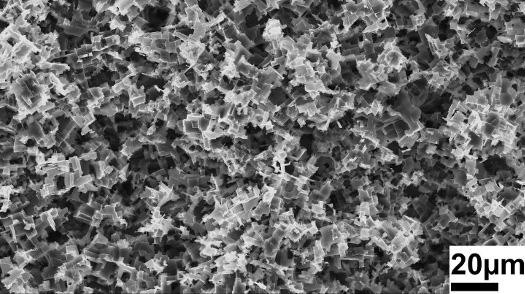
The targeted etching process of "nanoscale-sculpturing" roughens the upper layer of metal (here aluminum, 20 µm = 0.02 mm), creating a 3D structure with tiny hooks. A surface treated with this process can interlock like a 3D puzzle with the surfaces of almost all other materials, forming unbreakable bonds. With this method, it is even possible to create bonds between aluminum and copper. [Photo/Copyright: Melike Baytekin-Gerngroß]
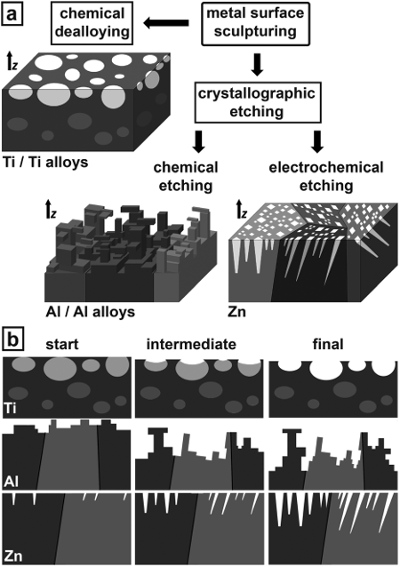
Schematic images of sculptured metal surfaces. (a) Strategy for sculpturing metal surfaces, and (b) the time evolution of the metal surface during sculpturing. For Ti, the dark grey areas indicate the Ti bulk matrix. The mid grey spots correspond to parts being electrochemically more instable compared to the bulk Ti matrix, but are inaccessible to preferential dissolution unlike the light grey ones. For Al and Zn, the diverse grey shades indicate different grain orientations, the black lines grain boundaries. [Source: Nanoscale Horizon]
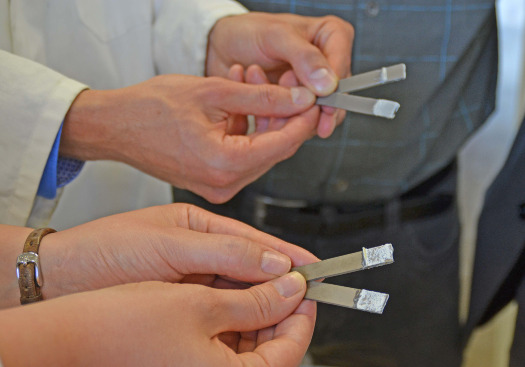
Sandblasted aluminum plates (background) cannot be glued successfully. The two glued plates separate at the interface between glue and metal; notice there is no white glue residue visible on one of the two plates. The aluminum plates (foreground) were treated with the new etching process before being glued. These plates could be separated, but the white glue particles left on both plates demonstrate that the bond between metal and glue is not broken; what broke is the glue itself. [Photo/Copyright: Julia Siekmann/Kiel University]
Surfaces with multifunctional properties
In addition, the research team exposed the hooked connections to extreme heat and moisture, to simulate weather conditions. This also did not affect their stability. Carstensen emphasized: "Our connections are extremely robust and weather resistant." A beneficial side effect of the process is that the etching makes the surfaces of metal water repellent. The resulting hook structure functions like a closely interlocked 3D labyrinth, without holes that can be penetrated by water. The metals therefore possess a kind of built-in corrosion protection.
"We actually don't know this kind of behavior from metals like aluminum. A lotus effect with pure metals -- i.e., without applying a water-repellent coating -- that is new," said Adelung. Using the etching process on its own on a metal, the laborious cleaning of surfaces, such as the pre-treatment of ships' hulls before they can be painted, could be rendered unnecessary, because the surface itself would become water resistant.
Adelung told Designfax that the largest etched metal surface so far was about .5 m2, but that the process is easily scalable and has the potential to even be done as a green (environmentally safe) process using different chemical etching materials.
Potentially limitless applications
"The range of potential applications is extremely broad, from metalworking industries such as ship building or aviation, to printing technology and fire protection, right through to medical applications," said Gerngroß. Because the "nanoscale-sculpturing" process not only creates a 3D surface structure, the targeted etching can also remove harmful particles from the surface, which is of great interest particularly for medical technology.
Titanium is often used for medical implants. To mechanically fix the titanium in place, small quantities of aluminum are added. However, the aluminum can trigger undesirable side effects in the body. "With our process, we can remove aluminum particles from the surface layer, and thereby obtain a significantly purer surface, which is much more tolerable for the human body. Because we only etch the uppermost layer on a micrometer scale, the stability of the whole implant remains unaffected," explained Carstensen.
The researchers have so far applied for four patents for the process. Businesses have already shown substantial interest in the potential applications. "And our specialist colleagues in materials sciences have also reacted enthusiastically to our discoveries," said Adelung.
* M. Baytekin-Gerngross, M.D. Gerngross, J. Carstensen, and R. Adelung. "Making metal surfaces strong, resistant, and multifunctional by nanoscale-sculpturing." Nanoscale Horizon. DOI: 10.1039/C6NH00140H.
You can also download the paper as a PDF here. It is quite short and really helps explain the process through the photo examples included.
Source: Kiel University
Published September 2016
Rate this article
View our terms of use and privacy policy
